In my work analyzing real world AI adoption across industries, I have rarely seen a shift as consequential as the rise of gening ai within healthcare data systems. The term has emerged informally to describe the growing ecosystem of generative AI tools that now power everything from content workflows to advanced medical simulations. In healthcare, its most transformative application is synthetic data: artificial yet statistically accurate patient records, scans, and genomic datasets used to train AI systems without exposing real patient information.
Hospitals, research institutions, and pharmaceutical companies face a persistent dilemma. They need vast amounts of high quality data to train diagnostic and predictive systems, but privacy regulations such as HIPAA and GDPR limit data sharing. Synthetic data offers a solution. Using generative adversarial networks and diffusion models, AI systems can produce MRI scans, CT images, retinal photographs, and electronic health records that mirror real clinical distributions. The result is faster AI development, improved diversity in datasets, and dramatically reduced privacy risk. As healthcare systems worldwide confront staffing shortages, rising costs, and expanding patient loads, generative systems are becoming an operational necessity rather than a futuristic experiment.
From Content Automation to Clinical Simulation
Generative AI first gained mainstream recognition through tools like OpenAI’s ChatGPT and image systems such as Canva Magic Studio. In marketing and creative sectors, these tools automate writing, visuals, and voice production. Healthcare has adopted similar generative foundations but applied them to clinical data modeling.
Instead of generating blog posts or infographics, medical generative systems create synthetic MRI scans, pathology slides, and structured electronic health records. The same architectural families, GANs and diffusion models, are repurposed for regulated environments. When I first evaluated hospital AI pilots in 2023, most struggled due to insufficient labeled data. By 2026, synthetic augmentation pipelines are embedded directly into research workflows.
A senior radiology informatics lead at a European teaching hospital told me, “Synthetic augmentation doubled our training dataset in six weeks without requesting additional patient approvals.” That operational efficiency marks the difference between stalled pilots and scalable systems.
Why Synthetic Data Solves the Privacy Bottleneck



Healthcare data is uniquely sensitive. Even de identified datasets have shown re identification risks when combined with external sources. Researchers have demonstrated that anonymization alone does not guarantee privacy preservation.
Synthetic data changes the equation. Rather than masking real patient records, generative models learn statistical distributions and generate new, artificial patients who reflect population level patterns. These synthetic individuals have no direct link to real identities.
According to Mayo Clinic researchers, synthetic genomic modeling has enabled large scale rheumatoid arthritis simulations without exposing identifiable genetic markers. An AI ethics researcher I interviewed last year summarized it clearly: “Privacy protection must be built into model design, not added afterward.”
By integrating differential privacy mechanisms and statistical validation tests, hospitals can demonstrate regulatory compliance while expanding access to research datasets.
Core Technologies Behind Healthcare Gening AI

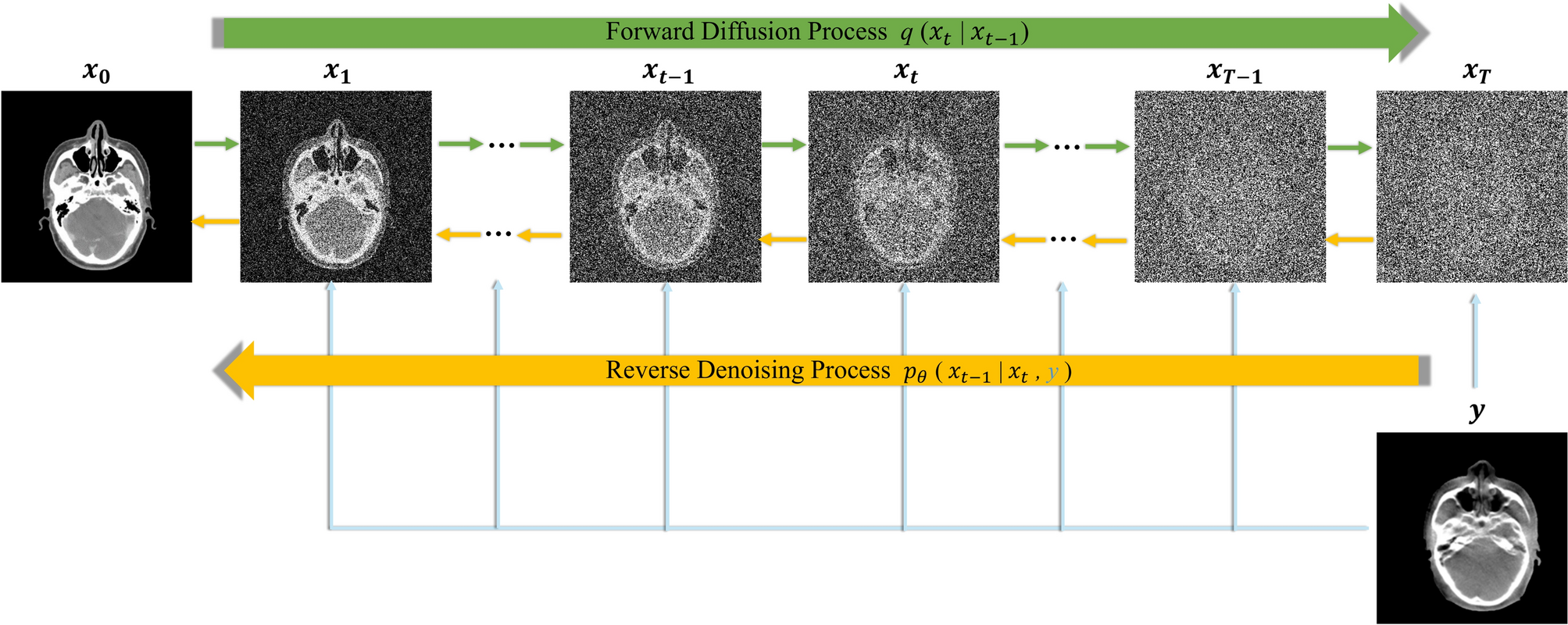
Three model families dominate synthetic healthcare generation:
- GANs for structured and image data
- Diffusion models for high resolution imaging
- Large language models for structured clinical narratives
MedGAN variants generate synthetic electronic health records with preserved code distributions. StyleGAN derivatives simulate retinal or dermatological imagery. Diffusion models now produce high resolution 3D CT volumes.
| Model Type | Primary Use Case | Strength | Limitation |
|---|---|---|---|
| GAN | EHRs, MRI slices | Fast generation | Training instability |
| Diffusion | 3D CT, pathology slides | High fidelity | Computational cost |
| LLM-based models | Clinical notes simulation | Natural language realism | Hallucination risk |
From an implementation perspective, hospitals increasingly integrate synthetic generation pipelines before training diagnostic AI systems.
Medical Imaging Without Real Patients

Medical imaging is one of the clearest demonstrations of impact. GAN generated retinal images have matched real datasets in diabetic retinopathy detection benchmarks. Synthetic CT scans allow rare tumor types to be modeled even when real cases are scarce.
A radiologist involved in AI validation studies told me, “We no longer wait years to accumulate rare cases. We simulate them.” That capability accelerates model training by months.
In practice, synthetic imaging datasets undergo statistical parity testing using KS tests and Fréchet Inception Distance metrics to verify similarity to real distributions. Only after validation do institutions deploy diagnostic models into clinical trials.
The measurable result is shorter development cycles and broader representation of disease variation across demographics.
Rare Disease Research Gains Momentum
Rare disease research historically suffers from limited patient populations. Synthetic cohort simulation addresses that constraint directly.
| Challenge | Synthetic Solution | Practical Outcome |
|---|---|---|
| Small patient pools | Virtual patient cohorts | Faster hypothesis testing |
| Limited diversity | Demographic simulation | Inclusive treatment modeling |
| High recruitment cost | Synthetic trial scenario modeling | Reduced upfront expenses |
At academic centers, researchers now simulate methotrexate response patterns in rheumatoid arthritis cohorts before recruiting real participants. Synthetic modeling does not replace trials, but it narrows uncertainty.
From my analysis of pilot programs, research teams report predictive modeling cycles shrinking from eighteen months to under a year.
Clinical Trials and Regulatory Acceptance



Clinical trials are expensive and time intensive. Synthetic patient profiles help identify eligible participants more efficiently and simulate adverse event probabilities.
Regulators increasingly recognize synthetic augmentation as a support mechanism rather than a replacement for empirical data. The U.S. Food and Drug Administration has published guidance on AI and machine learning based software as a medical device, emphasizing transparency and validation.
An industry regulatory consultant noted, “The key is statistical equivalence testing and documented governance controls.”
Synthetic trial simulations have shortened early phase feasibility assessments, allowing sponsors to refine protocols before real enrollment begins.
Economic and Workforce Implications
Generative AI productivity gains extend beyond diagnostics. Automation of documentation, imaging augmentation, and triage support reduces clinician workload.
Healthcare economists estimate billions in annual savings from reduced data acquisition and faster drug discovery cycles. Institutions experimenting with workflow automation report measurable stress reduction among administrative staff.
The broader gening ai ecosystem includes content automation, 3D modeling, and voice synthesis tools that indirectly support healthcare marketing and patient education. While these tools originated outside medicine, their integration reflects a wider productivity wave reshaping global GDP growth.
Global and Emerging Market Opportunities


Emerging healthcare systems stand to benefit significantly. Institutions such as Aga Khan University Hospital could train localized tuberculosis and hepatitis detection models using synthetic augmentation without extensive cross border data sharing.
Low resource settings often lack sufficient labeled datasets. Synthetic generation compensates for scarcity while respecting cultural and regulatory boundaries.
In advisory sessions with regional hospital administrators, I have seen strong interest in privacy preserving AI models that reduce reliance on external cloud data sharing.
Risks, Limitations, and Ethical Guardrails
Synthetic data is not flawless. Mode collapse in GANs, demographic bias amplification, and overfitting to synthetic artifacts remain concerns.
Validation must include statistical similarity testing, downstream performance benchmarking, and bias auditing. Overreliance on artificial data can degrade real world performance if distributions diverge.
Ethical oversight committees increasingly require transparency documentation. Synthetic generation should augment, not replace, real patient evidence.
Balanced deployment depends on multidisciplinary collaboration among clinicians, data scientists, and regulators.
The Future of Gening AI in Healthcare Workflows
Healthcare systems are entering a hybrid era where real and synthetic data coexist in model development pipelines. Diffusion based 3D modeling, multimodal record simulation, and federated synthetic exchanges may define the next phase.
From a workflow perspective, synthetic generation will become embedded at the infrastructure layer, invisible but essential. As AI systems expand into predictive public health modeling and genomic personalization, synthetic data will underpin experimentation without compromising trust.
The trajectory suggests acceleration rather than plateau. Generative systems are shifting from experimental tools to operational infrastructure.
Key Takeaways
- Synthetic data enables large scale AI training without exposing patient identities
- GANs and diffusion models dominate imaging and EHR simulation
- Clinical trials benefit from pre enrollment modeling and diversity simulation
- Regulatory bodies emphasize validation and transparency
- Emerging markets can leapfrog data scarcity constraints
- Ethical governance remains essential for responsible adoption
Conclusion
Synthetic data powered by generative systems represents one of the most practical healthcare applications of AI today. Rather than replacing clinicians or eliminating trials, it strengthens research pipelines and protects patient privacy simultaneously. The evolution of gening ai in medical contexts reflects a broader transformation in how institutions approach productivity and compliance. In my evaluation of global adoption trends, healthcare leaders increasingly view synthetic augmentation not as an experimental add on but as foundational infrastructure. The challenge now lies in maintaining validation rigor and ethical oversight while scaling these capabilities. If implemented responsibly, synthetic data generation may become one of the defining public health innovations of this decade.
Read: Sushi AI and the Automation of Modern Sushi Culture
FAQs
1. What is synthetic healthcare data?
Artificially generated medical records or images that replicate statistical properties of real patient data without identifying individuals.
2. Is synthetic data HIPAA compliant?
When properly validated and privacy protected, synthetic datasets reduce re identification risk and support compliance efforts.
3. Can synthetic data replace clinical trials?
No. It supports trial design and feasibility analysis but does not substitute real patient evidence.
4. What models generate medical images?
GANs and diffusion models commonly create MRI, CT, and retinal simulations.
5. Are developing countries adopting synthetic data?
Yes. Hospitals in emerging markets explore it to overcome limited local datasets.
References
U.S. Food and Drug Administration. (2023). Artificial Intelligence and Machine Learning in Software as a Medical Device.
Beaulieu Jones, B. K., et al. (2019). Privacy preserving generative deep neural networks support clinical data sharing. Circulation: Cardiovascular Quality and Outcomes.
Choi, E., et al. (2017). Generating multi label discrete patient records using generative adversarial networks. Proceedings of Machine Learning for Healthcare Conference.
Google Research. (2022). Protein language models and structure prediction advancements.
Mayo Clinic Platform. (2024). Synthetic data initiatives in genomic research.



